Check out a sample QA here. Want to see the full answer.
Ionic And Covalent Bonds Polar
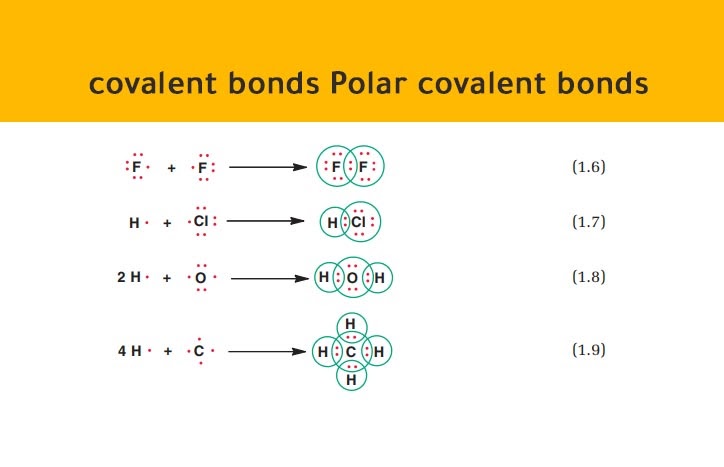
B C - Cl.

. C F - F. Atoms to the right of the periodic. NH3 NH NH HCN CO NO NO a.
Which of the following bonds is the most polar. Which of the following pairs of atoms are bound by a nonpolar covalent bond. Which one of the following is the most polar bond.
Apr 11 2015. Who are the experts. Out of O-F and N-F N-F bond is more polar as electronegativity of N is less than O and F is most electronegative.
What is the name of NH3. Solve any question of The p-Block Elements with-. Greater the electronegativity difference more is the polarity of the covalent bond formed by the elements.
Acetic acid is a polar molecule and can form hydrogen bonds with water molecules. Ο Ο Non-polar covalent bonds is a common bonding pattern for the noble gases. Yet acetic acid is also soluble in benzene C6H6 a nonpolar solvent that lacks the ability to form.
The polar character arises due to the differen in electronegativity. Polar covalent bond is existing if the electronegativity difference. O-H has the most polar bond kaso wala sa pagpipilian eh.
Which of the following bonds is most polar. 2 on a question. P-F S-F N-F O O-F.
Solve any question of Chemical Bonding and Molecular Structure with-. Report flag outlined. All are equally polar By signing up youll get thousands.
Hence the correct option is C Solve any question of Chemical Bonding and Molecular Structure with-. Which of the following bonds is the most polar. A polar covalent bond is formed with oxygen being negative and sulfur being positive.
H-F is more polar because F is more electronegative atom and where H View the full answer. P-F S-F N-F O O-F Expert Solution. Which of the following statements is correct.
Therefore C F bond will be the most polar. Kaya nga parang madami questions na wala sa pag pipilihan ang sagut. C Si As Cl Sr.
Which of the following bonds is the most polar. Want to see the full answer. The most polar bond will be the one with the highest electronegativity difference between its atoms ie C F bond.
The answer is b N - H. D Rb - Br. Hence it is most polar bond.
Electronegativity of N is approximately equal to Cl. The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. Between atom is same or much less than 04.
The quick answer - right from the get-go since nitrogen is one of the most electronegative elements in the periodic table the bond it forms with hydrogen will be the most polar out of all those listed. A non-polar covalent bond is formed. 4 In the bond between oxygen symbol O and sulfur symbol S.
Polarity that bond and also molecular geometry room the two factors that affect. Which of the following is not a type of covalent bond. The oxygen becomes a negative ion and the sulfur becomes a positive ion.
The electronegativity difference of N-P bond is maximum so it more polar bond. Which of the following bonds is the MOST polar. Correct option is C The electronegativity difference is highest between C and F.
Experts are tested by Chegg as specialists in their subject area. E All bonds have equal polarity. Therefore it has high solubility in water.
What is the molecular shape of CHBr3. Fluorine has highest electronegativity and iodine is least electronegative amongst group 17 elements so the electronegativity difference is highest in IF. A P - S.
Atoms bonded ionically have equal electronegativities. Which element has the greatest electronegativity. Thus N-Cl and N-N bonds are almost non-polar.
Which of the following bonds is most polar. The shape of NH4 is best described by. Polar covalent bonds deserve to be current in a nonpolar molecule.
Which species is not planar. In a polar covalent bond on atom is more electronegative than another and has a stronger hold on the shared electrons. B-C S-O C-O B-O C-C.



0 Comments